How Metals Displace Hydrogen From Water 35+ Pages Explanation in Google Sheet [1.4mb] - Updated
Get 40+ pages how metals displace hydrogen from water answer in Doc format. When potassium is added to water the metal melts and floats. I hope yuo get the best understanding from this my contribution. Here is a picture of the activity series. Read also hydrogen and how metals displace hydrogen from water The least reactive metals are not able to displace hydrogen from either of these and are therefore found at the bottom of the series.
Alkali metals displace hydrogen from water forming bases due to the fact that a their ionisation potential is less than that of other metals b they contain only one electron in their valence shell C they are far above the hydrogen in electro-chemical series based on oxidation potential d they are far below the hydrogen in electrochemical series based on oxidation potential. Metals more reactive than hydrogen normally displace hydrogen from water and acids to form base or salt.

Gcse Bined Chemistry Paper 1 Content Basic Notes Revision Yougotthis Chemistry Chemistry Paper Revision Papers Chemistry Revision This reaction can be easily represented by the following word equation.
| Topic: Heated magnesium displaces hydrogen from steam while aluminium zinc and iron will only do so at red heat. Gcse Bined Chemistry Paper 1 Content Basic Notes Revision Yougotthis Chemistry Chemistry Paper Revision Papers Chemistry Revision How Metals Displace Hydrogen From Water |
| Content: Explanation |
| File Format: DOC |
| File size: 3mb |
| Number of Pages: 27+ pages |
| Publication Date: July 2021 |
| Open Gcse Bined Chemistry Paper 1 Content Basic Notes Revision Yougotthis Chemistry Chemistry Paper Revision Papers Chemistry Revision |
 |
Most active easily oxidized readily lose electrons lithium.

The shed arrived early on the day they said it would arrive. Such metals will react with steam to form metal oxide and hydrogen gas. 11The alkali metals Li Na K Rb Cs and Fr are the most reactive metals in the periodic table - they all react vigorously or even explosively with cold water resulting in the displacement of hydrogen. Reason A metal can displace hydrogen from water only if its reduction potential is less than that of hydrogen. 2All metals above hydrogen in the activity series will displace hydrogen from an acid. Also Know which metal or metals can displace hydrogen from water Select all that apply.
Metals Replace Hydrogen From Dilute Acids Whereas Non Metals Do Not Why Cbse Class 10 Science Learn Cbse Forum 9Only a few of the most reactive metals are able to displace hydrogen from water while a larger number will displace Hfrom an acid.
| Topic: Activity Series of Metals in Aqueous Solutions. Metals Replace Hydrogen From Dilute Acids Whereas Non Metals Do Not Why Cbse Class 10 Science Learn Cbse Forum How Metals Displace Hydrogen From Water |
| Content: Synopsis |
| File Format: PDF |
| File size: 1.6mb |
| Number of Pages: 25+ pages |
| Publication Date: May 2017 |
| Open Metals Replace Hydrogen From Dilute Acids Whereas Non Metals Do Not Why Cbse Class 10 Science Learn Cbse Forum |
 |

C10 2 Reactivity Series Igcse Aid Calcium Magnesium Chemistry Notes Alkali Metal 27Assertion Zinc and iron decompose steam whereas copper and mercury do not.
| Topic: 2K 2H_2O 2KOH H_2 2Na 2H_2O 2NaOH H_2 Calcium reacts less violently with water. C10 2 Reactivity Series Igcse Aid Calcium Magnesium Chemistry Notes Alkali Metal How Metals Displace Hydrogen From Water |
| Content: Summary |
| File Format: PDF |
| File size: 1.9mb |
| Number of Pages: 40+ pages |
| Publication Date: July 2018 |
| Open C10 2 Reactivity Series Igcse Aid Calcium Magnesium Chemistry Notes Alkali Metal |
 |

Reactivity Of Metals Metal Displacement And The Activity Series Redox Reaction With Video Lessons In 2021 Teaching Chemistry Chemistry Study Guide Study Chemistry The higher reactive metals like sodium and potassium react with cold water vigorously.
| Topic: 2Metals donate electrons just like hydrogen does while non metals dont. Reactivity Of Metals Metal Displacement And The Activity Series Redox Reaction With Video Lessons In 2021 Teaching Chemistry Chemistry Study Guide Study Chemistry How Metals Displace Hydrogen From Water |
| Content: Solution |
| File Format: PDF |
| File size: 6mb |
| Number of Pages: 30+ pages |
| Publication Date: April 2019 |
| Open Reactivity Of Metals Metal Displacement And The Activity Series Redox Reaction With Video Lessons In 2021 Teaching Chemistry Chemistry Study Guide Study Chemistry |
 |

On Chemistry It took no time at all to set up.
| Topic: The hydrogen ignites instantly. On Chemistry How Metals Displace Hydrogen From Water |
| Content: Learning Guide |
| File Format: DOC |
| File size: 2.8mb |
| Number of Pages: 30+ pages |
| Publication Date: November 2017 |
| Open On Chemistry |
 |

Chem1 Electrochemistry Cell Potentials And Thermodynamics Chemistry Textbook Science Chemistry Electrochemistry It depends on the number of electrons in the outer amount of energy.
| Topic: 31The metals that are able to displace hydrogen from water and acids vary in their degree of activity. Chem1 Electrochemistry Cell Potentials And Thermodynamics Chemistry Textbook Science Chemistry Electrochemistry How Metals Displace Hydrogen From Water |
| Content: Summary |
| File Format: DOC |
| File size: 810kb |
| Number of Pages: 20+ pages |
| Publication Date: September 2021 |
| Open Chem1 Electrochemistry Cell Potentials And Thermodynamics Chemistry Textbook Science Chemistry Electrochemistry |
 |

Materials Metals And Non Metals Class 8 Extra Questions Science Chapter 4 Learn Cbse Extraquestionsfo Teaching Chemistry Study Chemistry Chemistry Lessons Some metals such as zinc and iron do not react with cold water but they do react with steam.
| Topic: Thats why hydrogen is also sometimes included in reactivity series of metals. Materials Metals And Non Metals Class 8 Extra Questions Science Chapter 4 Learn Cbse Extraquestionsfo Teaching Chemistry Study Chemistry Chemistry Lessons How Metals Displace Hydrogen From Water |
| Content: Answer Sheet |
| File Format: PDF |
| File size: 2.8mb |
| Number of Pages: 10+ pages |
| Publication Date: November 2017 |
| Open Materials Metals And Non Metals Class 8 Extra Questions Science Chapter 4 Learn Cbse Extraquestionsfo Teaching Chemistry Study Chemistry Chemistry Lessons |
 |

How Does The Metal Reactivity Series Work Example Chemistry Education Chemistry Lessons Biology Facts Potassium and sodium react vigorously with cold water to displace hydrogen while calcium reacts slowly.
| Topic: Zinc Zn Magnesium Mg These two metals which will displace. How Does The Metal Reactivity Series Work Example Chemistry Education Chemistry Lessons Biology Facts How Metals Displace Hydrogen From Water |
| Content: Synopsis |
| File Format: DOC |
| File size: 2.8mb |
| Number of Pages: 55+ pages |
| Publication Date: May 2018 |
| Open How Does The Metal Reactivity Series Work Example Chemistry Education Chemistry Lessons Biology Facts |
 |

Reactivity Series Reactivity Of Chemistry Basics Chemistry Lessons Chemistry Study Guide Metal Steam Metal Oxide Hydrogen Gas.
| Topic: Lead and copper do not displace hydrogen in any reaction at all. Reactivity Series Reactivity Of Chemistry Basics Chemistry Lessons Chemistry Study Guide How Metals Displace Hydrogen From Water |
| Content: Learning Guide |
| File Format: PDF |
| File size: 3.4mb |
| Number of Pages: 6+ pages |
| Publication Date: January 2017 |
| Open Reactivity Series Reactivity Of Chemistry Basics Chemistry Lessons Chemistry Study Guide |
 |

C10 2 Reactivity Series Igcse Aid Calcium Magnesium Chemistry Notes Alkali Metal Many non-metals are strongly reactive while others are in no way reactive.
| Topic: Also Know which metal or metals can displace hydrogen from water Select all that apply. C10 2 Reactivity Series Igcse Aid Calcium Magnesium Chemistry Notes Alkali Metal How Metals Displace Hydrogen From Water |
| Content: Learning Guide |
| File Format: Google Sheet |
| File size: 5mb |
| Number of Pages: 13+ pages |
| Publication Date: February 2020 |
| Open C10 2 Reactivity Series Igcse Aid Calcium Magnesium Chemistry Notes Alkali Metal |
 |

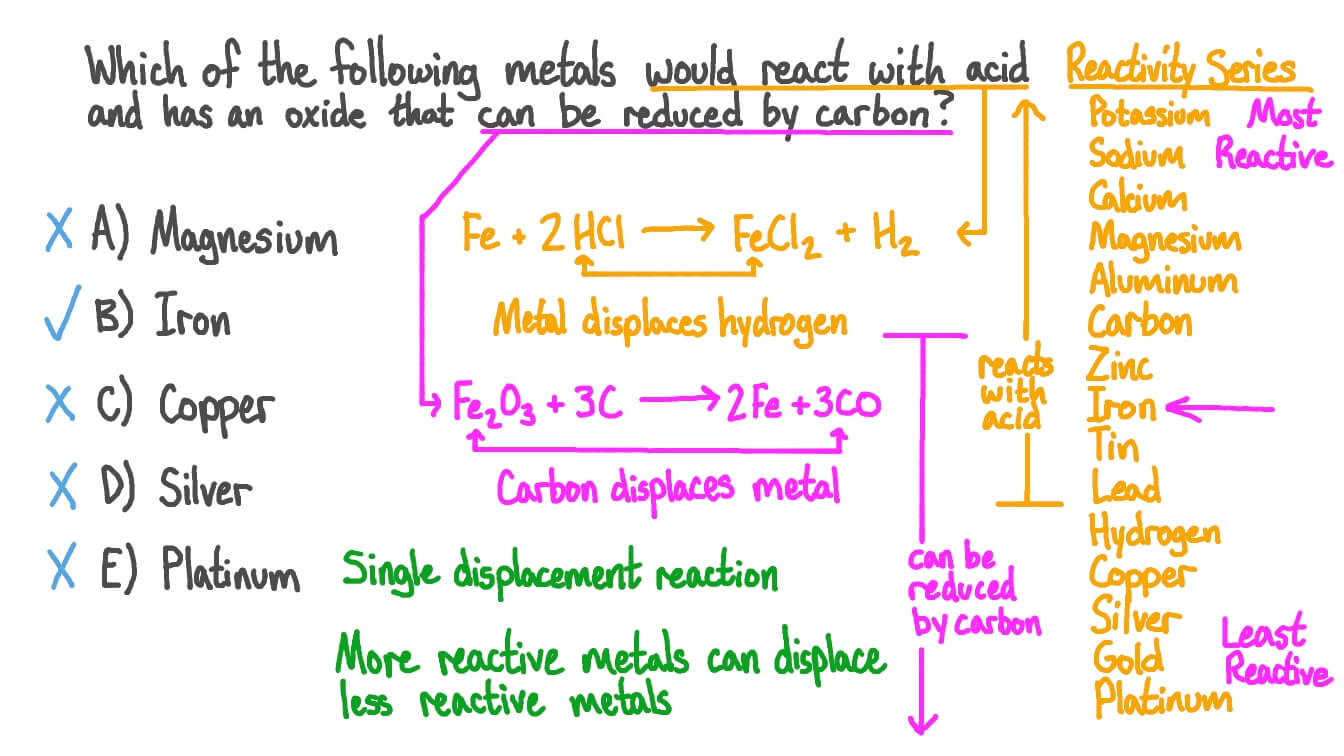
Question Video Identifying Which Metal Can React With Acid And Its Oxide Can Be Reduced Carbon Nagwa Such metals will react with steam to form metal oxide and hydrogen gas.
| Topic: The shed arrived early on the day they said it would arrive. Question Video Identifying Which Metal Can React With Acid And Its Oxide Can Be Reduced Carbon Nagwa How Metals Displace Hydrogen From Water |
| Content: Analysis |
| File Format: DOC |
| File size: 725kb |
| Number of Pages: 7+ pages |
| Publication Date: May 2020 |
| Open Question Video Identifying Which Metal Can React With Acid And Its Oxide Can Be Reduced Carbon Nagwa |
 |

Chemistry Science Reactivity Series Lesson Activities Chemistry Classroom Chemistry Education Chemistry Lessons
| Topic: Chemistry Science Reactivity Series Lesson Activities Chemistry Classroom Chemistry Education Chemistry Lessons How Metals Displace Hydrogen From Water |
| Content: Answer |
| File Format: PDF |
| File size: 1.5mb |
| Number of Pages: 21+ pages |
| Publication Date: January 2021 |
| Open Chemistry Science Reactivity Series Lesson Activities Chemistry Classroom Chemistry Education Chemistry Lessons |
 |
Its definitely easy to get ready for how metals displace hydrogen from water C10 2 reactivity series igcse aid calcium magnesium chemistry notes alkali metal how does the metal reactivity series work example chemistry education chemistry lessons biology facts metals replace hydrogen from dilute acids whereas non metals do not why cbse class 10 science learn cbse forum gcse bined chemistry paper 1 content basic notes revision yougotthis chemistry chemistry paper revision papers chemistry revision question video identifying which metal can react with acid and its oxide can be reduced carbon nagwa chem1 electrochemistry cell potentials and thermodynamics chemistry textbook science chemistry electrochemistry materials metals and non metals class 8 extra questions science chapter 4 learn cbse extraquestionsfo teaching chemistry study chemistry chemistry lessons on chemistry

Post a Comment
Post a Comment